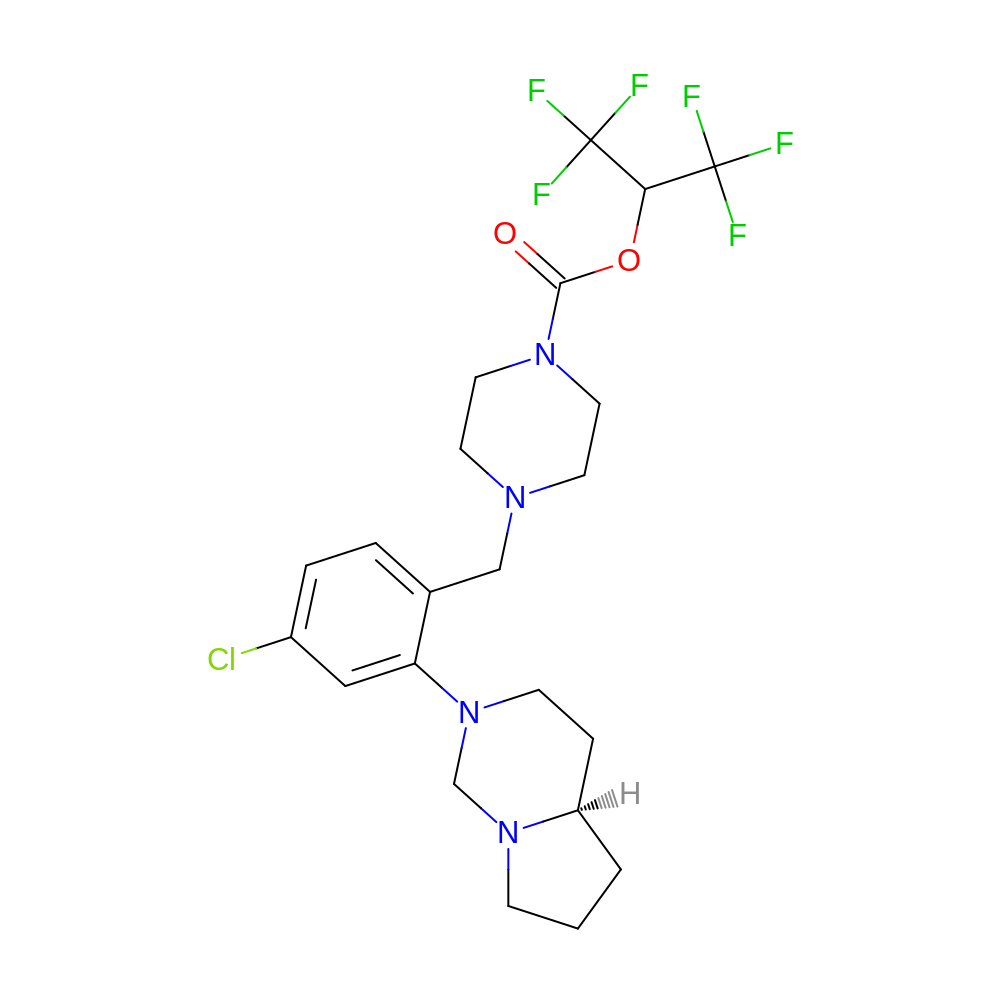
[2,2,2-trifluoro-1-(trifluoromethyl)ethyl] 4-[[2-[(4aR)-3,4,4a,5,6,7-hexahydro-1H-pyrrolo[1,2-c]pyrimidin-2-yl]-4-chloro-phenyl]methyl]piperazine-1-carboxylate
Inhibitor information
- CovInDB Inhibitor
- CI004699
- Name
- [2,2,2-trifluoro-1-(trifluoromethyl)ethyl] 4-[[2-[(4aR)-3,4,4a,5,6,7-hexahydro-1H-pyrrolo[1,2-c]pyrimidin-2-yl]-4-chloro-phenyl]methyl]piperazine-1-carboxylate
- Molecular Formula
- C22H27ClF6N4O2
- Molecular Weight
- 528.17 g/mol
- Structure
-
- IUPAC Name
- [2,2,2-trifluoro-1-(trifluoromethyl)ethyl] 4-[[2-[(4aR)-3,4,4a,5,6,7-hexahydro-1H-pyrrolo[1,2-c]pyrimidin-2-yl]-4-chloro-phenyl]methyl]piperazine-1-carboxylate
- InChI
- InChI=1S/C22H27ClF6N4O2/c23-16-4-3-15(18(12-16)33-7-5-17-2-1-6-32(17)14-33)13-30-8-10-31(11-9-30)20(34)35-19(21(24,25)26)22(27,28)29/h3-4,12,17,19H,1-2,5-11,13-14H2/t17-/m1/s1
- InChI Key
- QWEZIISMUXKJCH-QGZVFWFLSA-N
- Canonical SMILES
- O=C(OC(C(F)(F)F)C(F)(F)F)N1CCN(Cc2ccc(Cl)cc2N2CC[C@H]3CCCN3C2)CC1
- Cocrystal structures
- No cocrystal structures found for this inhibitor.
Calculated Properties
- Molecular Weight
-
528.17 g/mol
Computed by RDKit
- logP
-
4.93
Computed by ALOGPS
- logS
-
-3.77
Computed by ALOGPS
- Heavy Atom Count
-
35
Computed by RDKit
- Ring Count
-
4
Computed by RDKit
- Hydrogen Bond Acceptor Count
-
5
Computed by RDKit
- Hydrogen Bond Donor Count
-
0
Computed by RDKit
- Rotatable Bond Count
-
4
Computed by RDKit
- Topological Polar Surface Area
-
39.26 Å2
Computed by RDKit
3D Structure
Show Warhead
targets
| Name | ID | Warhead | Reaction Mechanism | Target Site | Activity Type | Relation | Value | Unit | Experiment Method | Assay | Reference |
|---|
Similar compounds in Virtual Screening library
Similar Natural compounds
No similar natural compounds found for this inhibitor.