Sotorasib
Drug information
- CovInDB Drug
- DB15569
- Name
- Sotorasib
- Molecular Formula
- C30H30F2N6O3
- Molecular Weight
- 560.2347 g/mol
- Description
- Sotorasib is an experimental KRAS inhibitor being investigated for the treatment of KRAS G12C mutant non small cell lung cancer, colorectal cancer, and appendix cancer.
- Status
- approved, investigational
- Structure
-
- Indication
- Sotorasib is indicated in the treatment of KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC) in adults who have received at least one prior systemic therapy.
- Mechanism of action
-
Normally GTP binds to KRAS, activating the protein and promoting effectors to the MAP kinase pathway.GTP is hydrolyzed to GDP, and KRAS is inactivated.KRAS G12C mutations impair hydrolysis of GTP, leaving it in the active form. Sotorasib binds to the cysteine residue in KRAS G12C mutations, holding the protein in its inactive form.The cysteine residue that sotorasib targets is not present in the wild type KRAS, which prevents off-target effects.This mutation is present in 13% of non small cell lung cancer, 3% of colorectal and appendix cancer, and 1-3% of solid tumors.
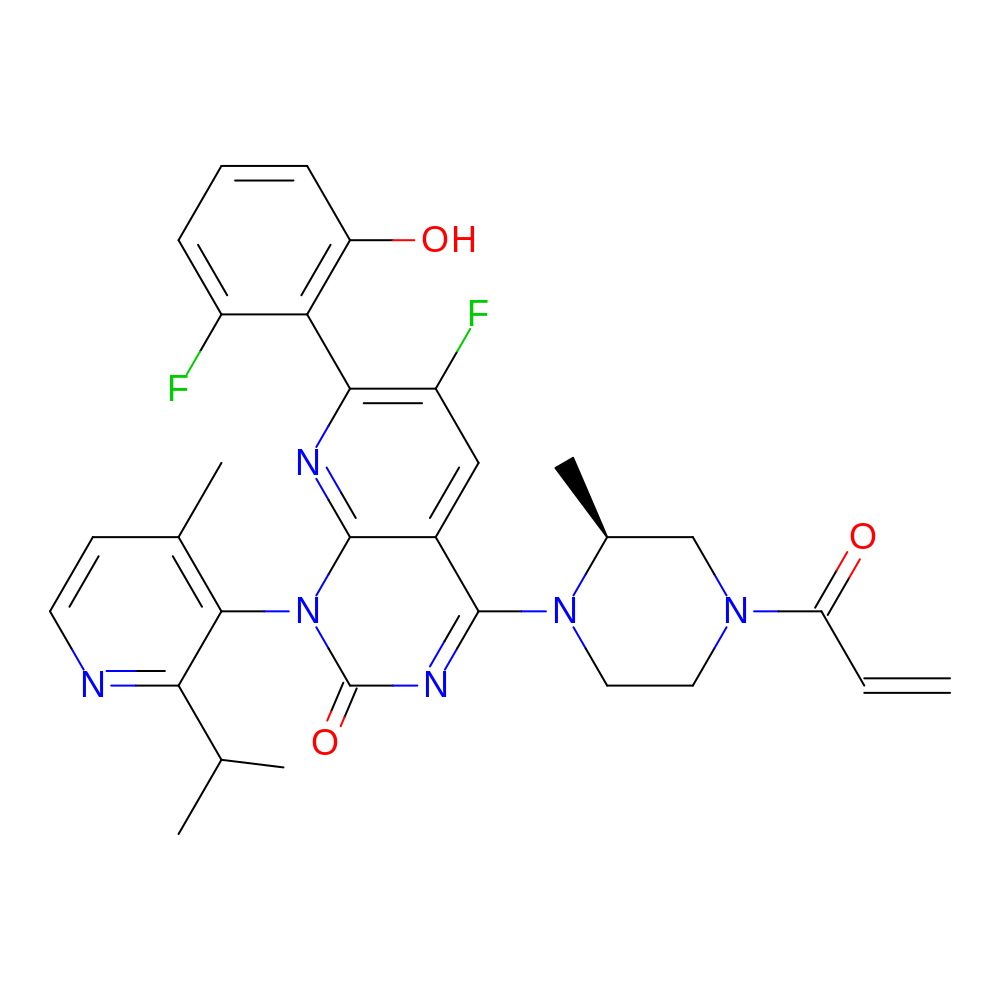
- IUPAC Name
- 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-[4-methyl-2-(propan-2-yl)pyridin-3-yl]-4-[(2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl]-1H,2H-pyrido[2,3-d]pyrimidin-2-one
- InChI
- InChI=1S/C30H30F2N6O3/c1-6-23(40)36-12-13-37(18(5)15-36)28-19-14-21(32)26(24-20(31)8-7-9-22(24)39)34-29(19)38(30(41)35-28)27-17(4)10-11-33-25(27)16(2)3/h6-11,14,16,18,39H,1,12-13,15H2,2-5H3/t18-/m0/s1
- InChI Key
- NXQKSXLFSAEQCZ-SFHVURJKSA-N
- Canonical SMILES
- CC(C)C1=NC=CC(C)=C1N1C(=O)N=C(N2CCN(C[C@@H]2C)C(=O)C=C)C2=CC(F)=C(N=C12)C1=C(O)C=CC=C1F
- Reference
- DrugBank
Covalent Inhibition
- Warhead
- Micheal Acceptor
- Target
-
GTPase KRas [ UniProt: P01116 ]
- Site
- CYS-12
- Inhibition Mechanism
-
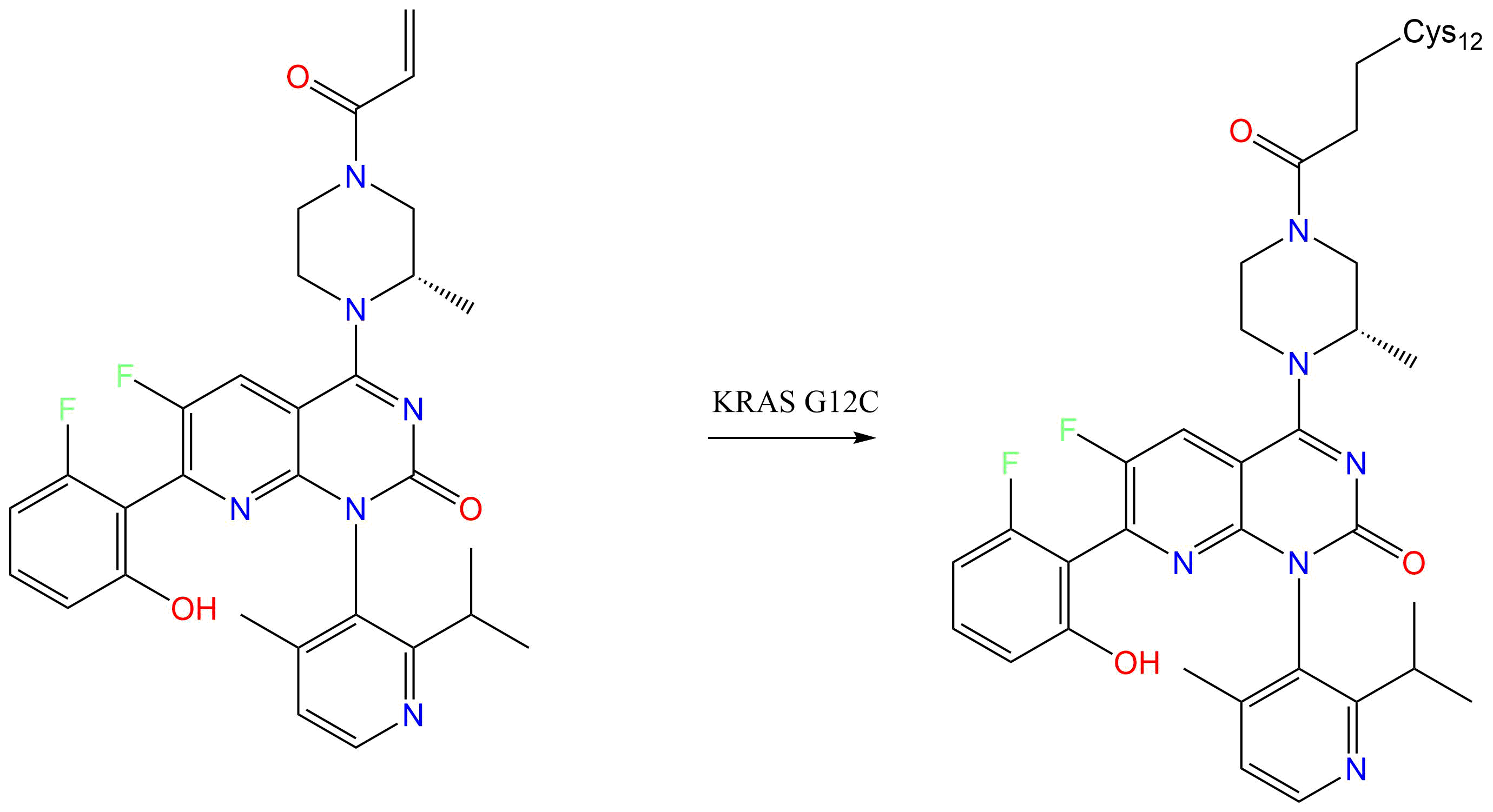
Discovery of a Covalent Inhibitor of KRAS(G12C) (AMG 510) for the Treatment of Solid Tumors.
3D Structure
Calculated Properties
- logP
-
3.6
Computed by ALOGPS
- logS
-
-4.48
Computed by ALOGPS
- Heavy Atom Count
-
41
Computed by RDKit
- Ring Count
-
5
Computed by RDKit
- Hydrogen Bond Acceptor Count
-
8
Computed by RDKit
- Hydrogen Bond Donor Count
-
1
Computed by RDKit
- Rotatable Bond Count
-
5
Computed by RDKit
- Topological Polar Surface Area
-
104.45 Å2
Computed by RDKit
Similar compounds in Virtual Screening library
Similar Natural compounds
No similar natural compounds found for this drug.