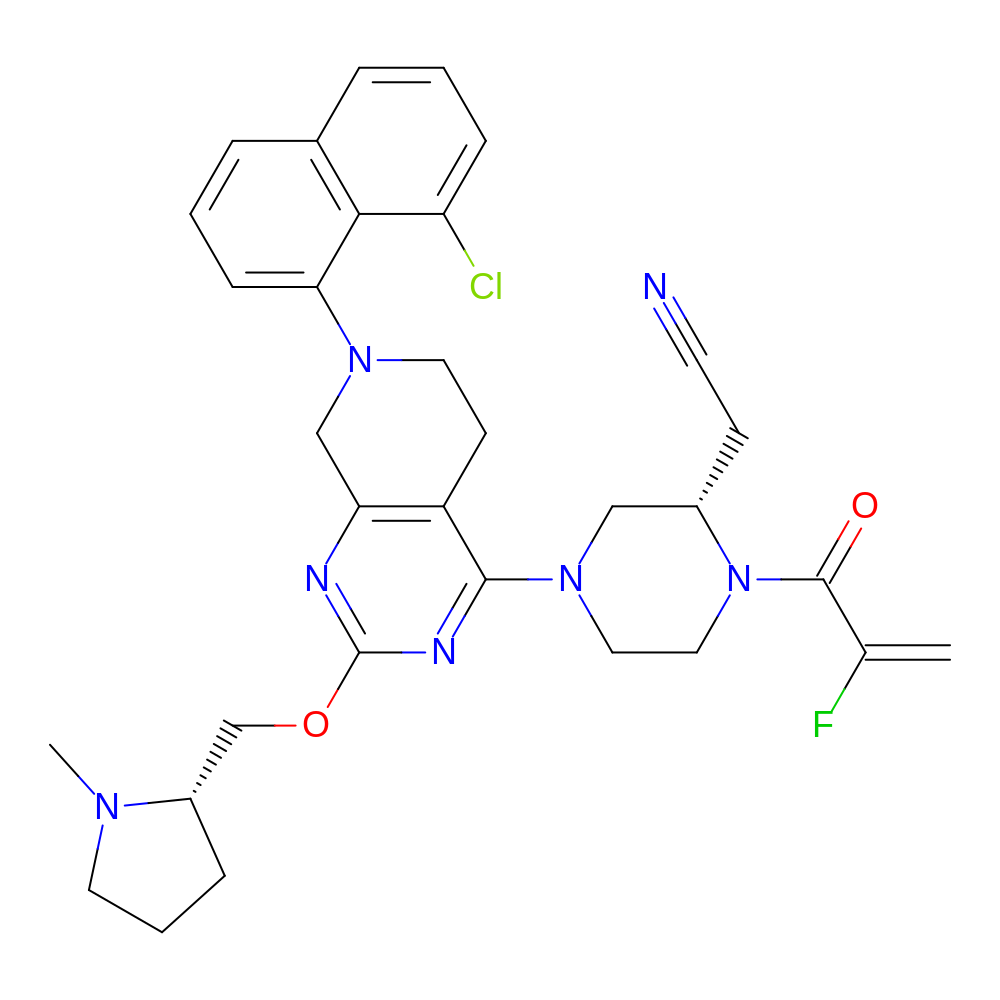
Adagrasib
Drug information
- CovInDB Drug
- DB15568
- Name
- Adagrasib
- Molecular Formula
- C32H35ClFN7O2
- Molecular Weight
- 603.2525 g/mol
- Description
- Adagrasib is a KRAS inhibitor indicated for the treatment of locally advanced or metastatic KRAS G12C-mutated non-small cell lung cancer in patients who have received at least one prior systemic therapy.
- Status
- approved, investigational
- Structure
-
- Indication
- Adagrasib is indicated for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), as determined by an FDA-approved test, who have received at least one prior systemic therapy. This indication is approved under accelerated approval based on objective response rate (ORR) and duration of response (DOR). Continued approval for this indication may be contingent upon verification and description of a clinical benefit in a confirmatory trial(s).
- Mechanism of action
-
In normal cells, KRAS is activated by binding to guanosine triphosphate (GTP), and this promotes the activation of the MAP kinase pathway and intracellular signal transduction. When GTP is hydrolyzed to guanosine diphosphate (GDP), KRAS is inactivated. This mechanism works as an "on"/"off" system that regulates cell growth. The substitution of Gly12 by cysteine in KRAS (KRASG12C) impairs GTP hydrolysis, and maintains KRAS in its active form. Therefore, the presence of this mutation leads to uncontrolled cellular proliferation and growth, as well as malignant transformation. Adagrasib is a covalent inhibitor of KRASG12C that irreversibly and selectively binds and locks KRASG12C in its inactive, guanosine diphosphate–bound state. Therefore, the use of adagrasib inhibits tumor cell growth and viability in cancers with KRASG12C mutations with minimal off-target activity.
- IUPAC Name
- 2-[(2S)-4-[7-(8-chloronaphthalen-1-yl)-2-{[(2S)-1-methylpyrrolidin-2-yl]methoxy}-5H,6H,7H,8H-pyrido[3,4-d]pyrimidin-4-yl]-1-(2-fluoroprop-2-enoyl)piperazin-2-yl]acetonitrile
- InChI
- InChI=1S/C32H35ClFN7O2/c1-21(34)31(42)41-17-16-40(18-23(41)11-13-35)30-25-12-15-39(28-10-4-7-22-6-3-9-26(33)29(22)28)19-27(25)36-32(37-30)43-20-24-8-5-14-38(24)2/h3-4,6-7,9-10,23-24H,1,5,8,11-12,14-20H2,2H3/t23-,24-/m0/s1
- InChI Key
- PEMUGDMSUDYLHU-ZEQRLZLVSA-N
- Canonical SMILES
- [H][C@@]1(COC2=NC3=C(CCN(C3)C3=CC=CC4=C3C(Cl)=CC=C4)C(=N2)N2CCN(C(=O)C(F)=C)[C@@]([H])(CC#N)C2)CCCN1C
- Reference
- DrugBank
Covalent Inhibition
- Warhead
- Micheal Acceptor
- Target
-
GTPase KRas [ UniProt: P01116 ]
- Site
- CYS-12
- Inhibition Mechanism
-

The KRASG12C Inhibitor, MRTX849, Provides Insight Toward Therapeutic Susceptibility of KRAS Mutant Cancers in Mouse Models and Patients.
3D Structure
Calculated Properties
- logP
-
5.16
Computed by ALOGPS
- logS
-
-4.72
Computed by ALOGPS
- Heavy Atom Count
-
43
Computed by RDKit
- Ring Count
-
6
Computed by RDKit
- Hydrogen Bond Acceptor Count
-
8
Computed by RDKit
- Hydrogen Bond Donor Count
-
0
Computed by RDKit
- Rotatable Bond Count
-
7
Computed by RDKit
- Topological Polar Surface Area
-
88.83 Å2
Computed by RDKit
Similar compounds in Virtual Screening library
Similar Natural compounds
No similar natural compounds found for this drug.