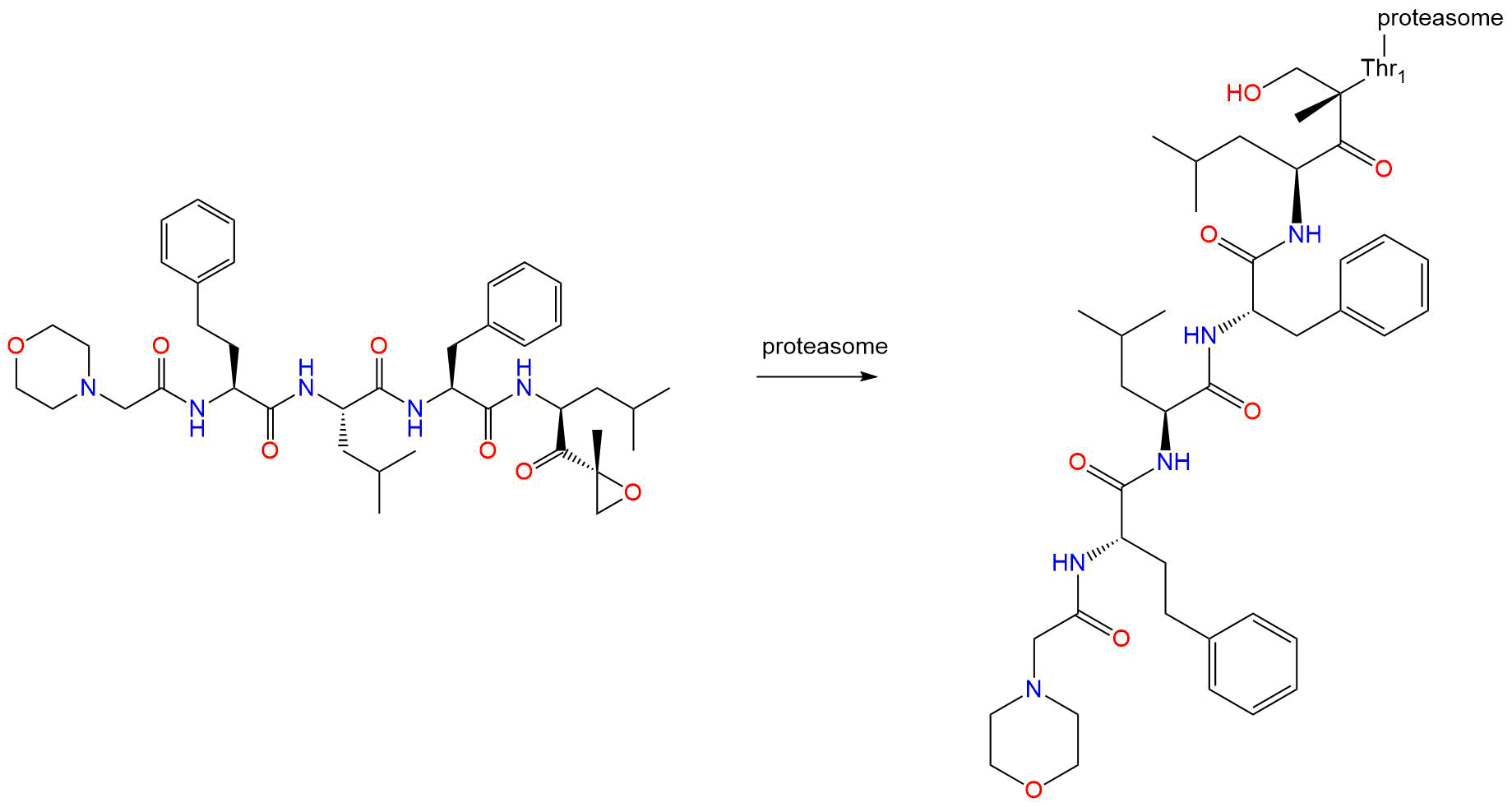
Carfilzomib
Drug information
- CovInDB Drug
- DB08889
- Name
- Carfilzomib
- Molecular Formula
- C40H57N5O7
- Molecular Weight
- 719.43 g/mol
- Description
- Carfilzomib is an injectable antineoplastic agent (IV only). Chemically, it is a modified tetrapeptidyl epoxide and an analog of epoxomicin. It is also a selective proteasome inhibitor. FDA approved on July 20, 2012.
- Status
- approved, investigational
- Structure
-
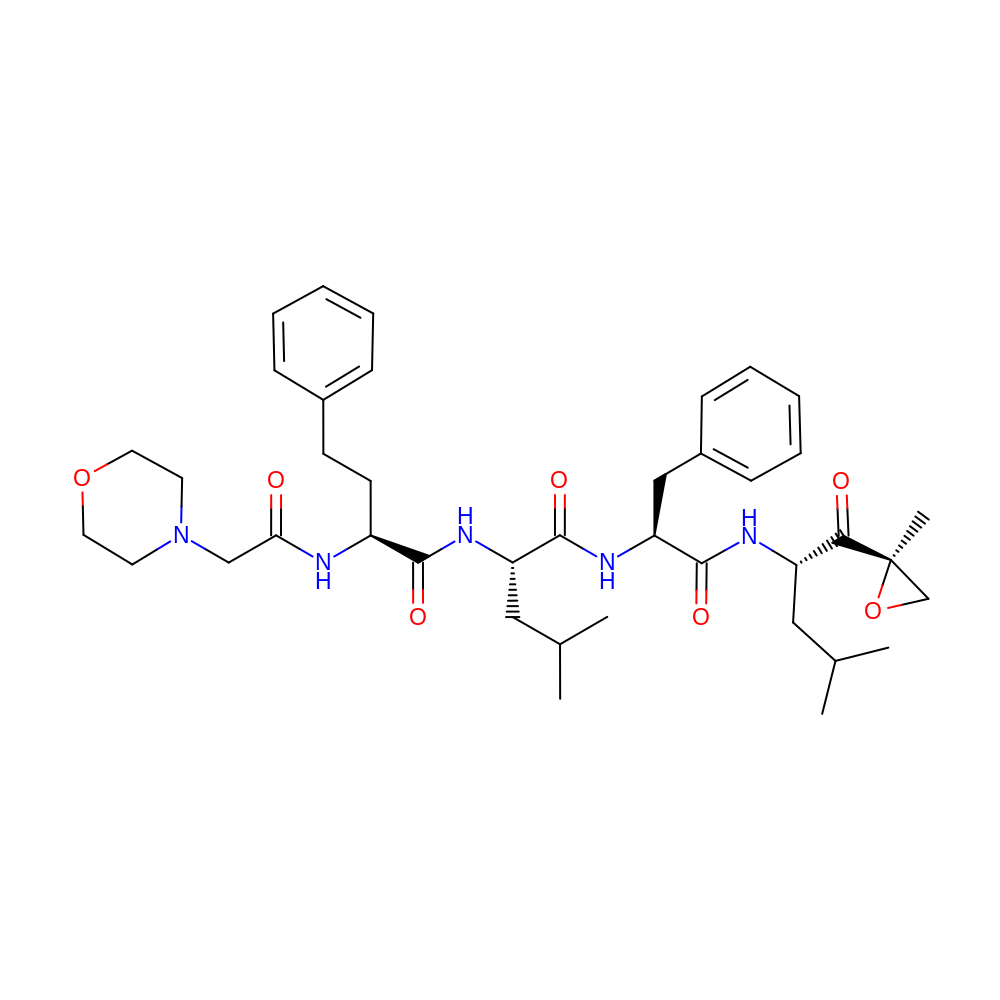
- Indication
- Carfilzomib is indicated for the treatment of patients with multiple myeloma who have received at least two prior therapies including bortezomib and an immunomodulatory agent and have demonstrated disease progression on or within 60 days of completion of the last therapy. Approval is based on response rate.
- Mechanism of action
-
Carfilzomib is made up of four modified peptides and acts as a proteasome inhibitor. Carfilzomib irreversibly and selectively binds to N-terminal threonine-containing active sites of the 20S proteasome, the proteolytic core particle within the 26S proteasome. This 20S core has 3 catalytic active sites: the chymotrypsin, trypsin, and caspase-like sites. Inhibition of the chymotrypsin-like site by carfilzomib (β5 and β5i subunits) is the most effective target in decreasing cellular proliferation, ultimately resulting in cell cycle arrest and apoptosis of cancerous cells. At higher doses, carfilzomib will inhibit the trypsin-and capase-like sites.
- IUPAC Name
- (2S)-N-[(1S)-1-benzyl-2-[[(1S)-3-methyl-1-[(2R)-2-methyloxirane-2-carbonyl]butyl]amino]-2-oxo-ethyl]-4-methyl-2-[[(2S)-2-[(2-morpholinoacetyl)amino]-4-phenyl-butanoyl]amino]pentanamide
- InChI
- InChI=1S/C40H57N5O7/c1-27(2)22-32(36(47)40(5)26-52-40)42-39(50)34(24-30-14-10-7-11-15-30)44-38(49)33(23-28(3)4)43-37(48)31(17-16-29-12-8-6-9-13-29)41-35(46)25-45-18-20-51-21-19-45/h6-15,27-28,31-34H,16-26H2,1-5H3,(H,41,46)(H,42,50)(H,43,48)(H,44,49)/t31-,32-,33-,34-,40+/m0/s1
- InChI Key
- BLMPQMFVWMYDKT-NZTKNTHTSA-N
- Canonical SMILES
- CC(C)C[C@H](NC(=O)[C@H](CCC1=CC=CC=C1)NC(=O)CN1CCOCC1)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1
- Reference
- DrugBank
Covalent Inhibition
3D Structure
Calculated Properties
- logP
-
3.21
Computed by ALOGPS
- logS
-
-5.17
Computed by ALOGPS
- Heavy Atom Count
-
52
Computed by RDKit
- Ring Count
-
4
Computed by RDKit
- Hydrogen Bond Acceptor Count
-
8
Computed by RDKit
- Hydrogen Bond Donor Count
-
4
Computed by RDKit
- Rotatable Bond Count
-
20
Computed by RDKit
- Topological Polar Surface Area
-
158.47 Å2
Computed by RDKit
Similar compounds in Virtual Screening library
Similar Natural compounds
No similar natural compounds found for this drug.