Selegiline
Drug information
- CovInDB Drug
- DB01037
- Name
- Selegiline
- Molecular Formula
- C13H17N
- Molecular Weight
- 187.14 g/mol
- Description
- A selective, irreversible inhibitor of Type B monoamine oxidase. It is used in newly diagnosed patients with Parkinson's disease. It may slow progression of the clinical disease and delay the requirement for levodopa therapy. It also may be given with levodopa upon onset of disability. (From AMA Drug Evaluations Annual, 1994, p385) The compound without isomeric designation is Deprenyl.
- Status
- approved, investigational, vet_approved
- Structure
-
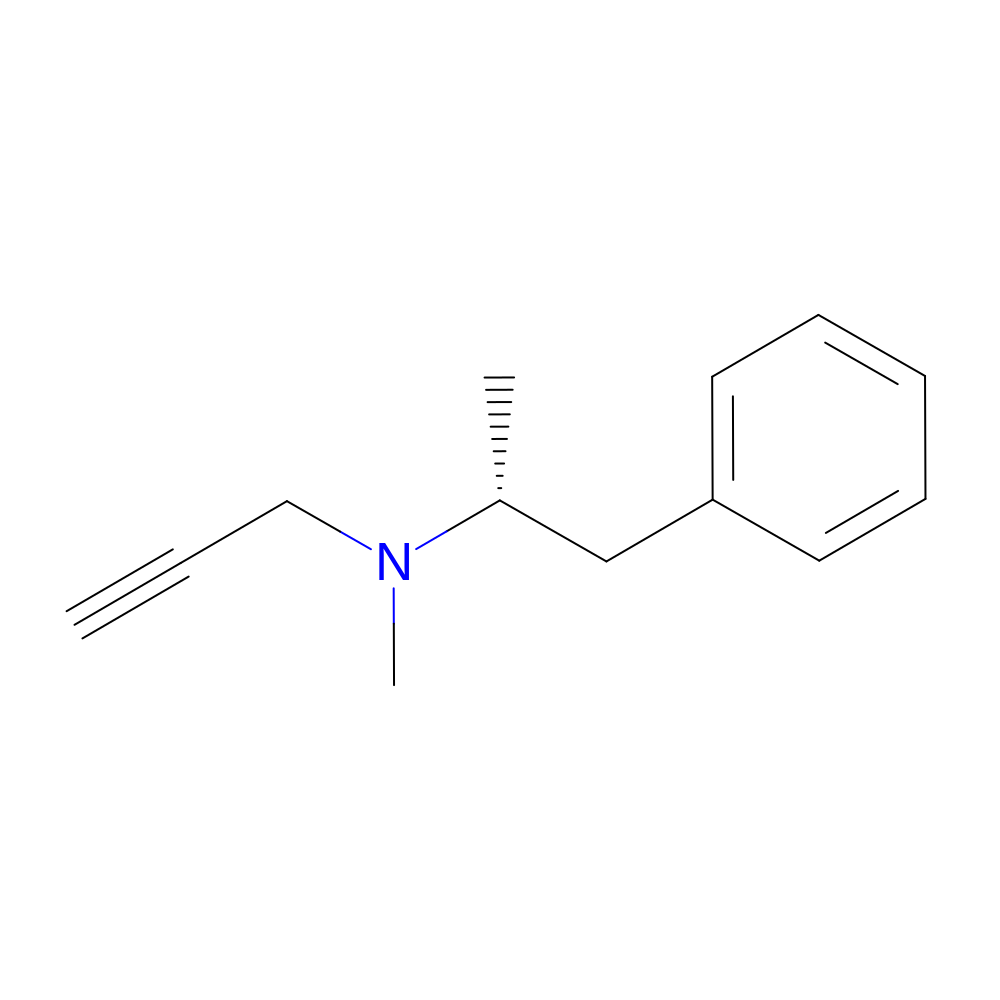
- Indication
- Monotherapy for initial treatment of Parkinson's disease, as well as an adjunct therapy in patients with a decreased response to levodopa/carbadopa. Also used for the palliative treatment of mild to moderate Alzheimer's disease and at higher doses, for the treatment of depression.
- Mechanism of action
-
Although the mechanisms for selegiline's beneficial action in the treatment of Parkinson's disease are not fully understood, the selective, irreversible inhibition of monoamine oxidase type B (MAO-B) is thought to be of primary importance. MAO-B is involved in the oxidative deamination of dopamine in the brain. Selegiline binds to MAO-B within the nigrostriatal pathways in the central nervous system, thus blocking microsomal metabolism of dopamine and enhancing the dopaminergic activity in the substantial nigra. Selegiline may also increase dopaminergic activity through mechanisms other than inhibition of MAO-B. At higher doses, selegiline can also inhibit monozmine oxidase type A (MAO-A), allowing it to be used for the treatment of depression.
- IUPAC Name
- (2R)-N-methyl-1-phenyl-N-prop-2-ynyl-propan-2-amine
- InChI
- InChI=1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/m1/s1
- InChI Key
- MEZLKOACVSPNER-GFCCVEGCSA-N
- Canonical SMILES
- C[C@H](CC1=CC=CC=C1)N(C)CC#C
- Reference
- DrugBank
Covalent Inhibition
- Warhead
- Alkynyl
- Target
-
MAO-B [ UniProt: P27338 ]
- Site
- FAD cofactor
- Inhibition Mechanism
-
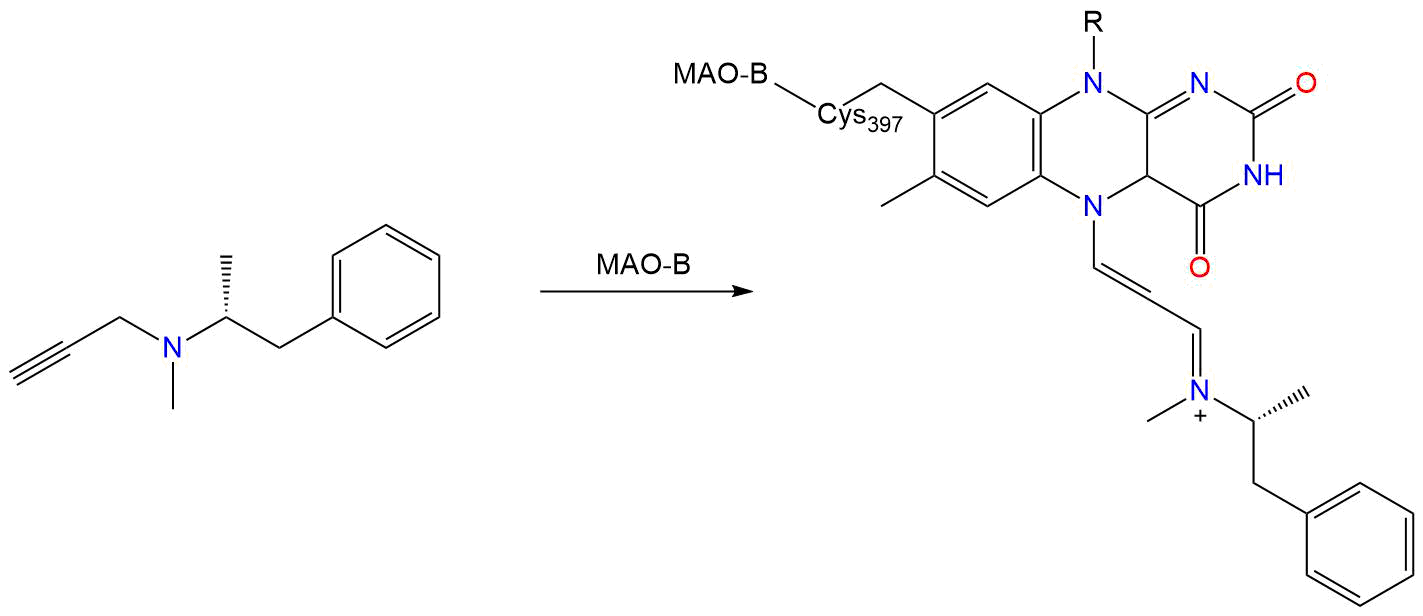
Crystal Structures of Monoamine Oxidase B in Complex with Four Inhibitors of the N-Propargylaminoindan Class
3D Structure
Calculated Properties
- logP
-
3.08
Computed by ALOGPS
- logS
-
-3.87
Computed by ALOGPS
- Heavy Atom Count
-
14
Computed by RDKit
- Ring Count
-
1
Computed by RDKit
- Hydrogen Bond Acceptor Count
-
1
Computed by RDKit
- Hydrogen Bond Donor Count
-
0
Computed by RDKit
- Rotatable Bond Count
-
4
Computed by RDKit
- Topological Polar Surface Area
-
3.24 Å2
Computed by RDKit
Similar compounds in Virtual Screening library
Similar Natural compounds
No similar natural compounds found for this drug.