7LZY
Target information
- RCSB PDB
- 7LZY
- Title
- Structure of SARS-CoV-2 3CL protease in complex with inhibitor 3c
- Method
- X-RAY DIFFRACTION
- Resolution
- 1.85
- Classification
- HYDROLASE/INHIBITOR
- Organism
- Severe acute respiratory syndrome coronavirus 2
- Protein
- Replicase polyprotein 1ab (P0DTD1) Looking for covalent inhibitors of this target ?
- Year
- 2021
- Publication Title
- Structure-Guided Design of Potent Inhibitors of SARS-CoV-2 3CL Protease: Structural, Biochemical, and Cell-Based Studies.
- Abstract
-
The COVID-19 pandemic is having a major impact on public health worldwide, and there is an urgent need for the creation of an armamentarium of effective therapeutics, including vaccines, biologics, and small-molecule therapeutics, to combat SARS-CoV-2 and emerging variants. Inspection of the virus life cycle reveals multiple viral- and host-based choke points that can be exploited to combat the virus. SARS-CoV-2 3C-like protease (3CLpro), an enzyme essential for viral replication, is an attractive target for therapeutic intervention, and the design of inhibitors of the protease may lead to the emergence of effective SARS-CoV-2-specific antivirals. We describe herein the results of our studies related to the application of X-ray crystallography, the Thorpe-Ingold effect, deuteration, and stereochemistry in the design of highly potent and nontoxic inhibitors of SARS-CoV-2 3CLpro.
- External Link
- RCSB PDB
Ligand information
- HET
- YMJ
- Chain ID
- A
- HET Number
- 401
- Molecular Formula
- C23H41N3O8S
- Structure
-
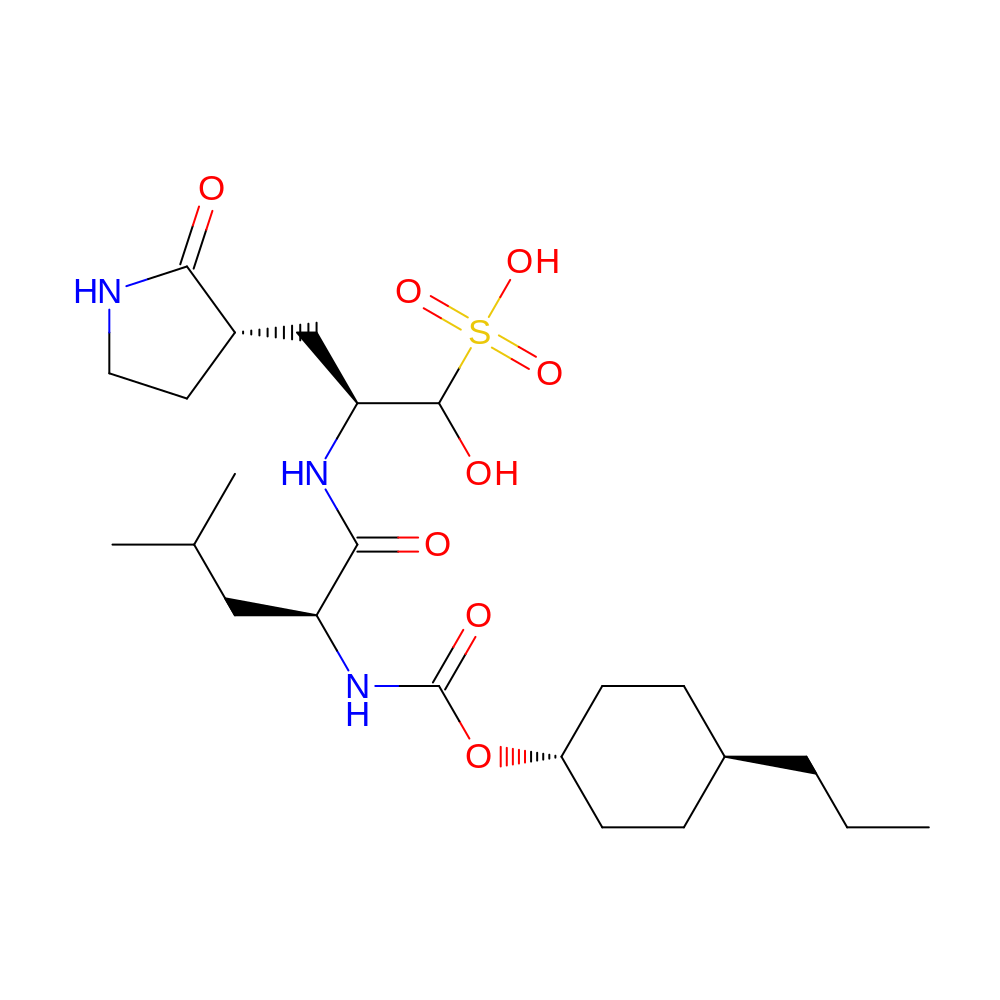
- IUPAC Name
- (2S)-1-hydroxy-2-[[(2S)-4-methyl-2-[(4-propylcyclohexoxy)carbonylamino]pentanoyl]amino]-3-[(3S)-2-oxopyrrolidin-3-yl]propane-1-sulfonic acid
- InChI
- InChI=1S/C23H41N3O8S/c1-4-5-15-6-8-17(9-7-15)34-23(30)26-18(12-14(2)3)21(28)25-19(22(29)35(31,32)33)13-16-10-11-24-20(16)27/h14-19,22,29H,4-13H2,1-3H3,(H,24,27)(H,25,28)(H,26,30)(H,31,32,33)/t15-,16-,17-,18-,19-,22?/m0/s1
- InChI Key
- VCQYBHSDNWQLAS-ZAWVYXTHSA-N
- Canonical SMILES
- CCC[C@H]1CC[C@H](OC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]2CCNC2=O)C(O)S(=O)(=O)O)CC1
- Bioactivity data
- CI006817
Covalent Binding
- Warhead
- Sulfonic acid
- Reaction Mechanism
- Nucleophilic Substitution
- Residue
- CYS : 145
- Residue Chain
- A
- Interactions
- Pharmacophore Model