4QWF
Target information
- RCSB PDB
- 4QWF
- Title
- yCP beta5-M45I mutant in complex with carfilzomib
- Method
- X-RAY DIFFRACTION
- Resolution
- 3.0
- Classification
- HYDROLASE/HYDROLASE INHIBITOR
- Organism
- Saccharomyces cerevisiae
- Protein
- Proteasome subunit beta type-1 (P38624)
- Year
- 2015
- Publication Title
- Bortezomib-Resistant Mutant Proteasomes: Structural and Biochemical Evaluation with Carfilzomib and ONX 0914.
- Abstract
-
Inhibition of the 20S proteasome by bortezomib (Velcade) constitutes a successfully applied therapy for blood cancer. However, emerging resistance restricts its medicinal use. For example, mutations in the proteolytically active β5-subunit of the proteasome, the main target of inhibitors, were reported to impair drug binding and thus to reduce therapeutic efficacy. Using yeast as a model system, we describe here a systematic evaluation of these mutations by cell growth analysis, proteasome inhibition assays, and X-ray crystallography. The 11 mutants examined display decreased proliferation rates, impaired proteolytic activity, and marked resistance to bortezomib as well as the α',β'-epoxyketone inhibitors carfilzomib (Kyprolis) and ONX 0914, while the second-generation compound carfilzomib was the least affected. In total, 49 proteasome X-ray structures, including structural data on proteasome-carfilzomib complexes, reveal three distinct molecular mechanisms that hamper both drug binding and natural substrate turnover to an extent that is still compatible with cell survival.
- External Link
- RCSB PDB
Ligand information
- HET
- 3BV
- Chain ID
- N
- HET Number
- 201
- Molecular Formula
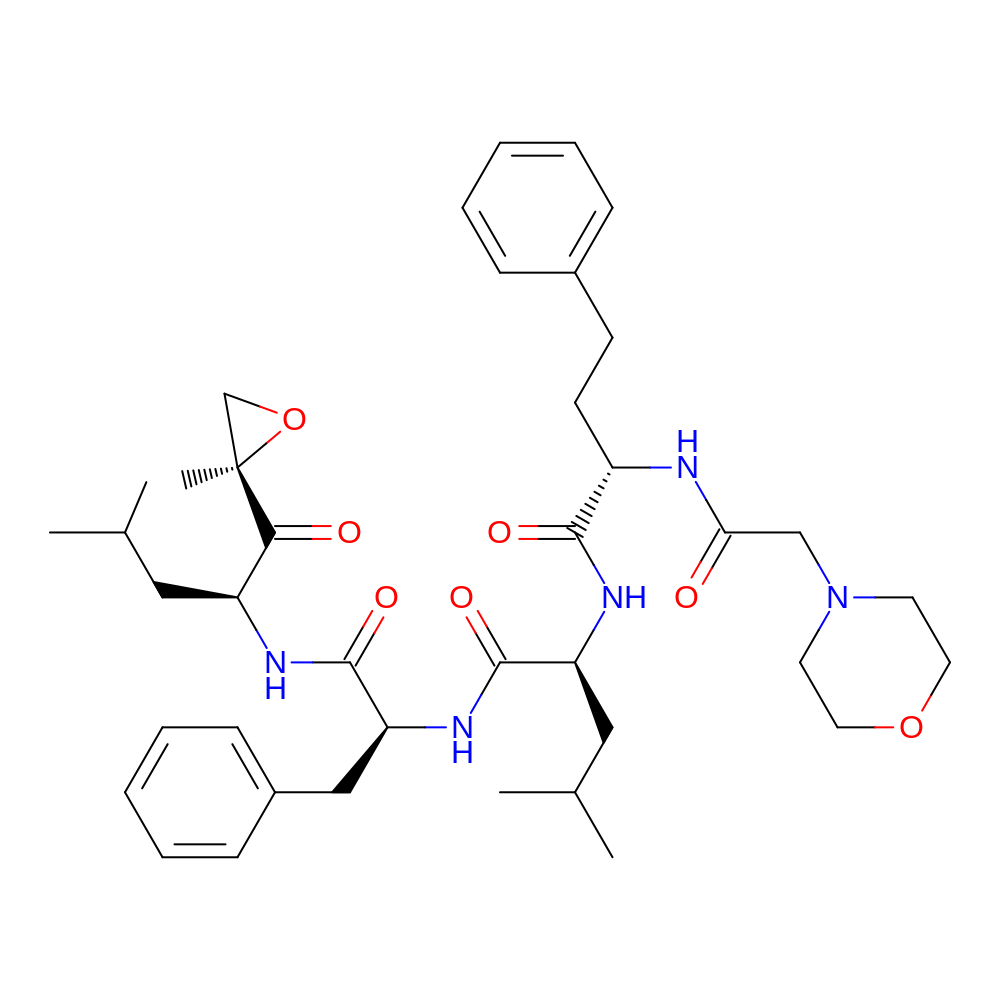
- C40H57N5O7
- Structure
-
- IUPAC Name
- (2S)-N-[(1S)-1-benzyl-2-[[(1S)-3-methyl-1-[(2R)-2-methyloxirane-2-carbonyl]butyl]amino]-2-oxo-ethyl]-4-methyl-2-[[(2S)-2-[(2-morpholinoacetyl)amino]-4-phenyl-butanoyl]amino]pentanamide
- InChI
- InChI=1S/C40H57N5O7/c1-27(2)22-32(36(47)40(5)26-52-40)42-39(50)34(24-30-14-10-7-11-15-30)44-38(49)33(23-28(3)4)43-37(48)31(17-16-29-12-8-6-9-13-29)41-35(46)25-45-18-20-51-21-19-45/h6-15,27-28,31-34H,16-26H2,1-5H3,(H,41,46)(H,42,50)(H,43,48)(H,44,49)/t31-,32-,33-,34-,40+/m0/s1
- InChI Key
- BLMPQMFVWMYDKT-NZTKNTHTSA-N
- Canonical SMILES
- CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)NC(=O)CN1CCOCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1
- Bioactivity data
- CI005157
Covalent Binding
- Warhead
- Carbonyl
- Reaction Mechanism
- Nucleophilic Addition
- Residue
- THR : 1
- Residue Chain
- N
- Interactions
- Pharmacophore Model